
In today’s fast-moving global economy, speed and compliance are critical competitive advantages. For medical device manufacturers, the United Arab Emirates offers a strategic hub for reaching global markets efficiently, backed by world-class infrastructure, regulatory alignment, and access to key trade corridors.
The UAE’s medical devices market was valued at USD 1.92 billion in 2023, with projected growth of 8.87% CAGR through 2030. Supported by initiatives like Make it in the Emirates, the country has positioned itself as a leader in healthcare innovation, making it an ideal base for SMEs and global exporters alike.
This guide outlines the essentials for exporting medical devices from the UAE, covering product definitions, regulatory requirements, documentation, compliance, and logistics.
What Counts as a Medical Device?
The UAE Ministry of Health and Prevention (MOHAP) defines a medical device as any instrument, apparatus, appliance, software, material, or article intended for human use. Key categories include:
- Diagnostic & monitoring equipment: MRI machines, blood glucose monitors, imaging software
- Dental & surgical instruments: From scalpels to robotic surgery systems
- AI-based software for medical use: Diagnostic or patient-management tools
- Implants & disposables: Pacemakers, artificial joints, sterile gloves, syringes
The UAE aligns with Global Harmonization Task Force (GHTF) principles, meaning devices compliant in the UAE are recognized internationally—a significant advantage for global exports.
Navigating UAE Regulatory Requirements
Compliance with MOHAP regulations is mandatory before export. Key steps include:
MOHAP Device Registration
- Manufacturer & Marketing Authorization Holder (MAH) Registration: Required for the company responsible for marketing the device.
- Product Classification: Devices are categorized by risk (Class A–D).
- Technical Dossier Submission: Detailed documentation covering design, manufacturing, and safety performance.
- Registration Certificate: Issued upon successful review and fee payment.
Local Authorized Representative
Manufacturers outside the UAE must appoint a licensed local representative to manage regulatory communications and maintain ongoing compliance.
Export Documentation Essentials
Accurate documentation ensures smooth customs clearance and reduces delays:
- Export Declaration: Filed electronically via platforms like Dubai Trade
- Certificate of Origin (CoO): Issued by the Dubai Chamber; may provide tariff benefits under UAE CEPA agreements
- MOHAP Free Sale Certificate: Confirms local compliance and aids acceptance in foreign markets
- Document Attestation: Some destinations require MOFAIC attestation and embassy legalization

HS Codes & Product Classification
Correct HS codes are essential for customs. Work with logistics experts like DHL Express to ensure accuracy.
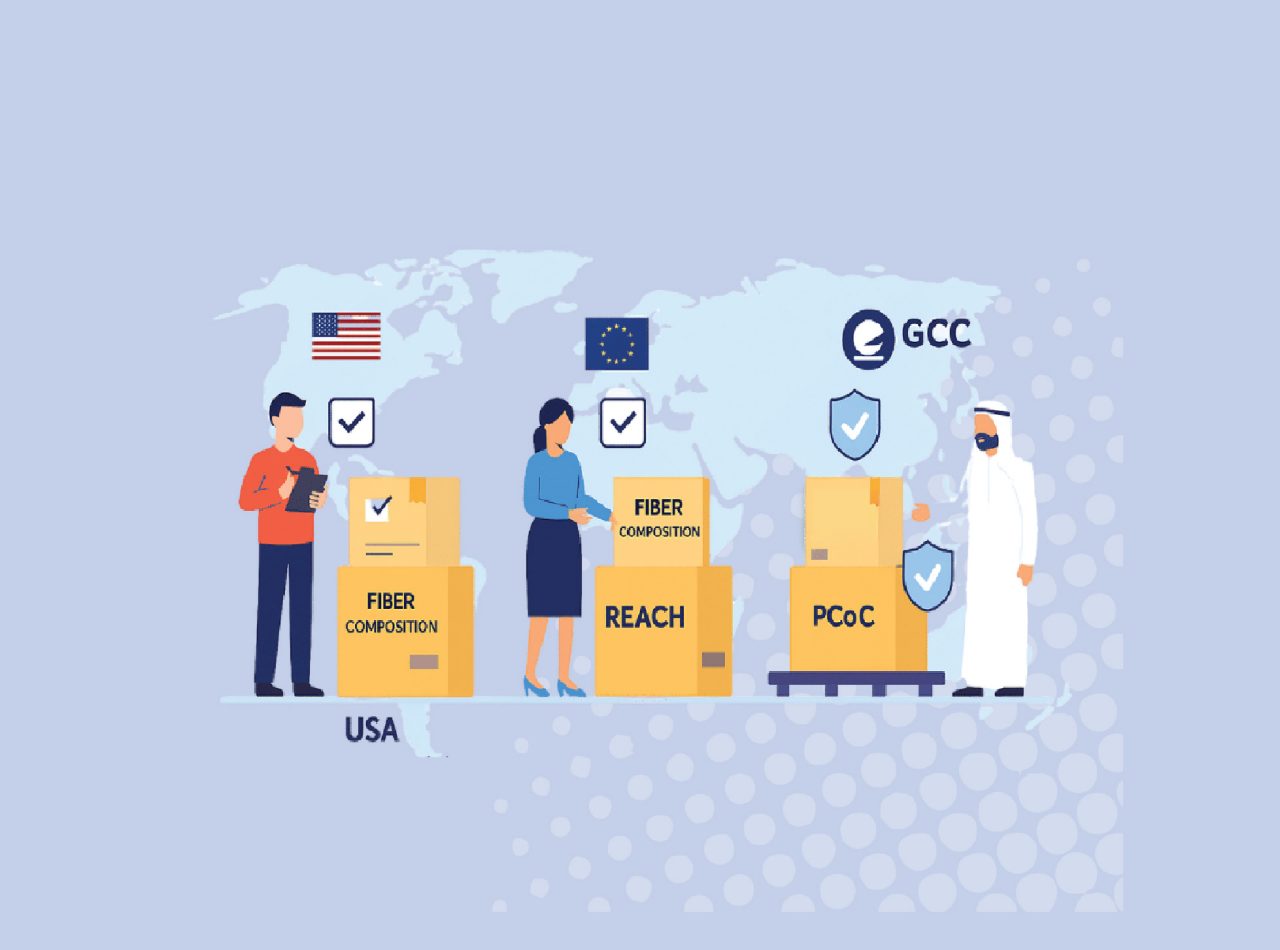
Compliance in Destination Markets
Exporters must adhere to local labeling, packaging, and safety standards:
Region | Labeling Requirements | Safety & Certification |
USA | Fiber content, origin, manufacturer, care instructions | Children’s products require CPSIA compliance |
EU | Fiber composition mandatory | REACH restricts certain chemicals |
GCC | Arabic or bilingual labels, origin, care instructions | PCoC from SASO-approved body may be required |
Always verify your device against restricted or dual-use lists (FANR, Dubai Customs) and consider WHO Prequalification for humanitarian or international aid markets.
Logistics: Ensuring Safe Delivery
Medical devices require specialized handling to maintain quality and integrity:
- Labeling & Packaging: Clear, accurate, and ISO-compliant labels (ISO 15223-1)
- Temperature-Controlled Shipments: Chilled or frozen products must maintain the cold chain per WHO guidelines
- Certified Logistics Partners: GDP-certified carriers ensure compliance with global quality standards
DHL Medical Express offers:
- 250+ GDP-qualified warehouses globally
- Ambient, chilled, and frozen transport
- Real-time shipment tracking and secure handling
From the UAE to the World
Exporting medical devices from the UAE demands careful attention to regulations, documentation, and logistics. Done correctly, it enables SMEs to reach international markets while upholding product safety and brand integrity.
Let us be your partner in global health. Discover how DHL Express can simplify your logistics and accelerate your international growth





